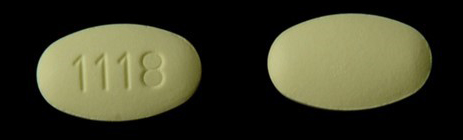
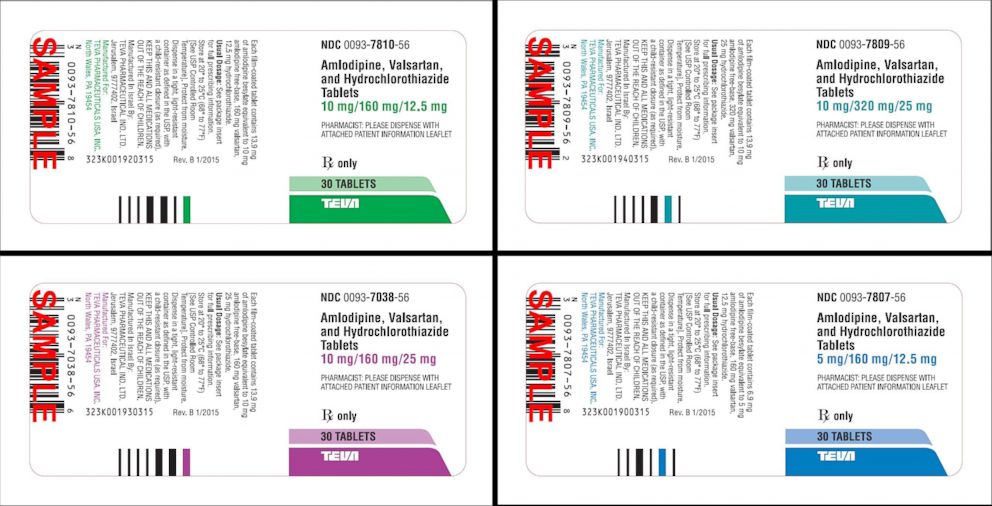
Teva Pharmaceuticals USA Issues Voluntary Nationwide Recall of all Amlodipine/Valsartan Combination Tablets and Amlodipine/Valsartan/Hydrochlorothiazide Combination Tablets that are Within Expiry | FDA

Government of the Virgin Islands - High Blood Pressure Treatment Drug Valsartan Recalled Healthcare professionals and patients are being advised of a voluntary recall of products containing the active pharmaceutical ingredient (API)

Teva's recall of U.S.-made drugs latest example of contamination fears in generic marketplace; report says Lilly, Pfizer and former Mylan plant in Morgantown have been cited in the past | WV News | wvnews.com

Teva Pharmaceuticals USA recalls Losartan Potassium 25mg and 100mg Tablets USP sold exclusively to Golden State Medical Supply - Pharmaceutical Business review





/cloudfront-us-east-1.images.arcpublishing.com/gray/UVAUY3N3VBKKPJZ24MSVDZL62I.png)