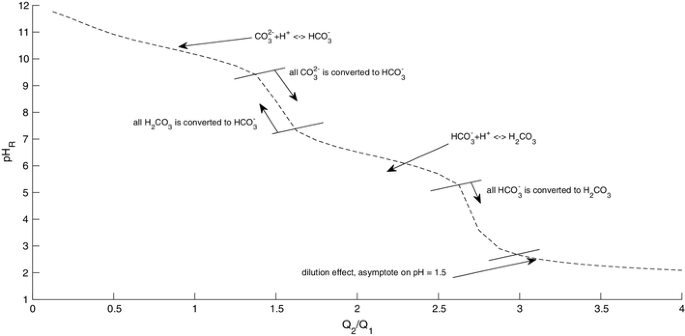
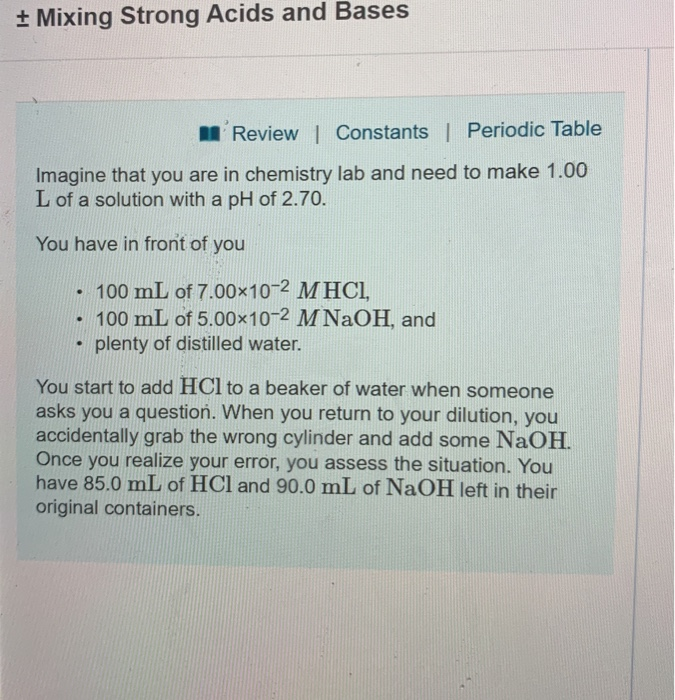
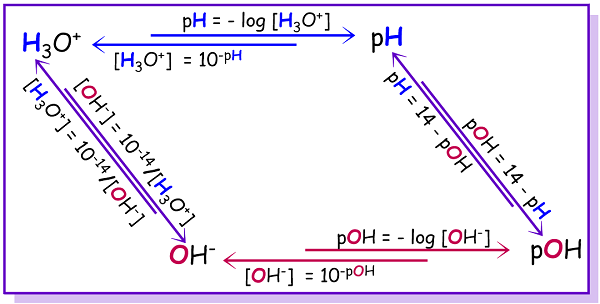
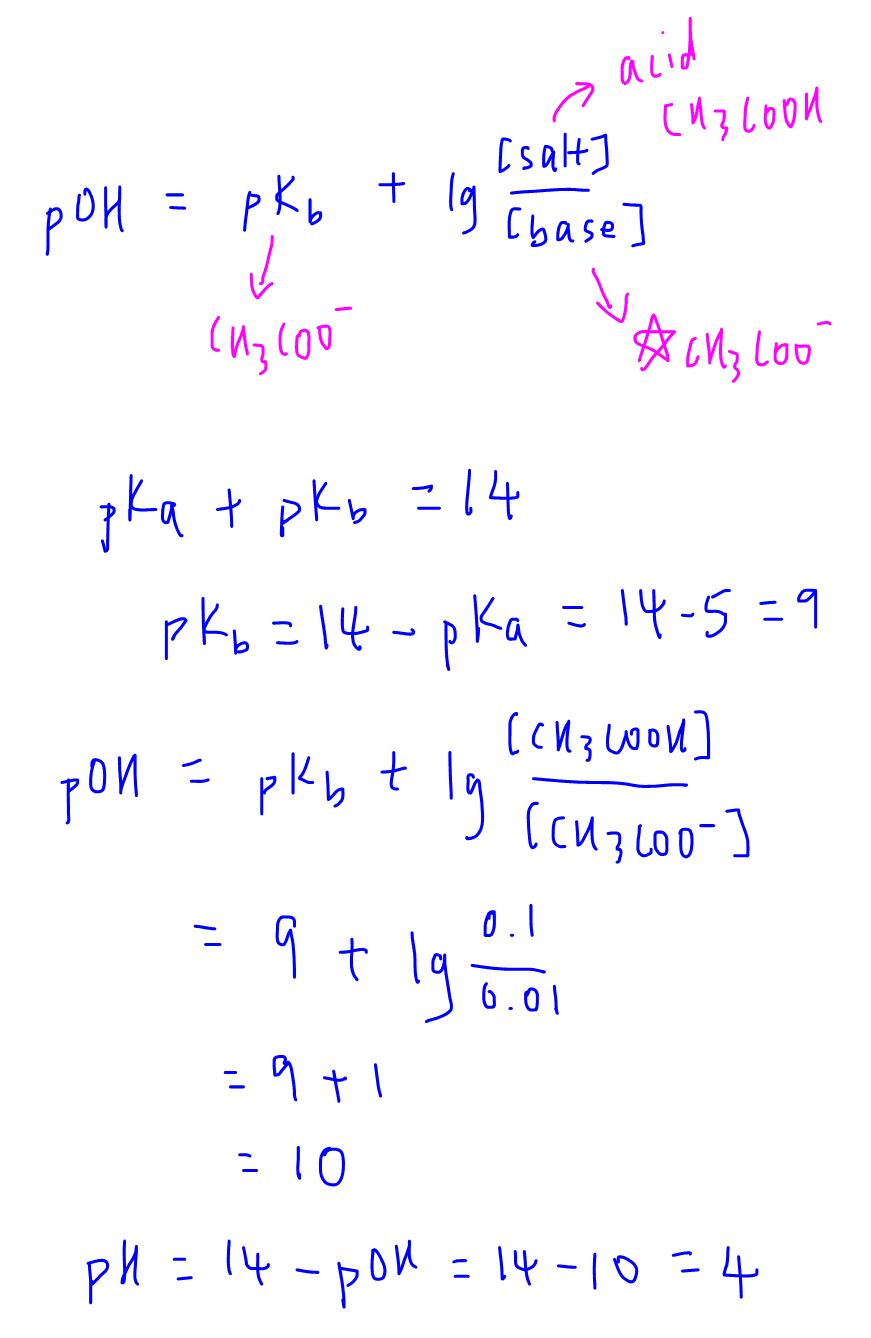Calculate the pH of a solution obtained by mixing equal volumes of the solutions with pH = 3 and pH = 5.

Flow chart for calculating the CO2 volume mixing ratio using the Raman... | Download Scientific Diagram

equilibrium - Calculation of the pH of a mixture of a strong acid and weak acid - Chemistry Stack Exchange
The equal volume of two HCL solutions of pH=3 and pH=5 were mixed. What is the pH of the resulting solution? - Quora
![The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ]. The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ].](https://d2rrqu68q7r435.cloudfront.net/images/4298277/0914b99c-8837-49a9-86f7-3cbcdb1ec4a6.jpg)
The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ].