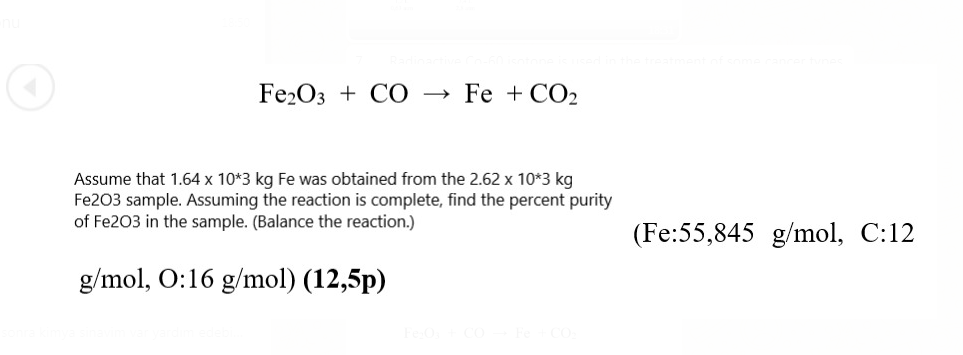
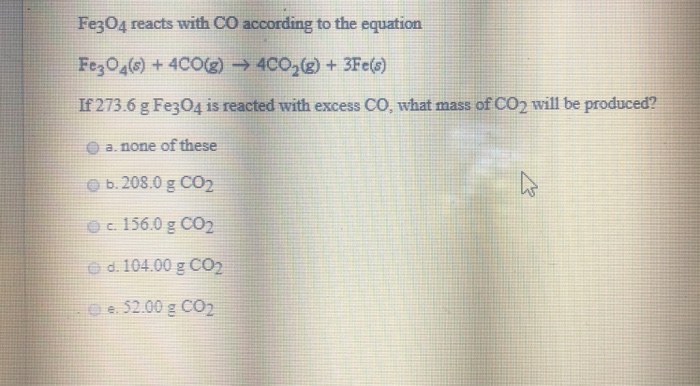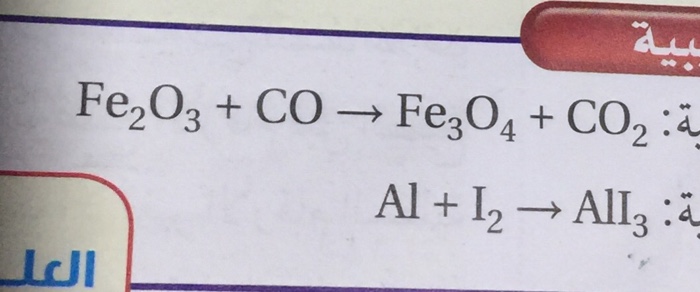Vibrational properties of CO2 adsorbed on the Fe3O4 (111) surface: Insights gained from DFT: The Journal of Chemical Physics: Vol 152, No 10

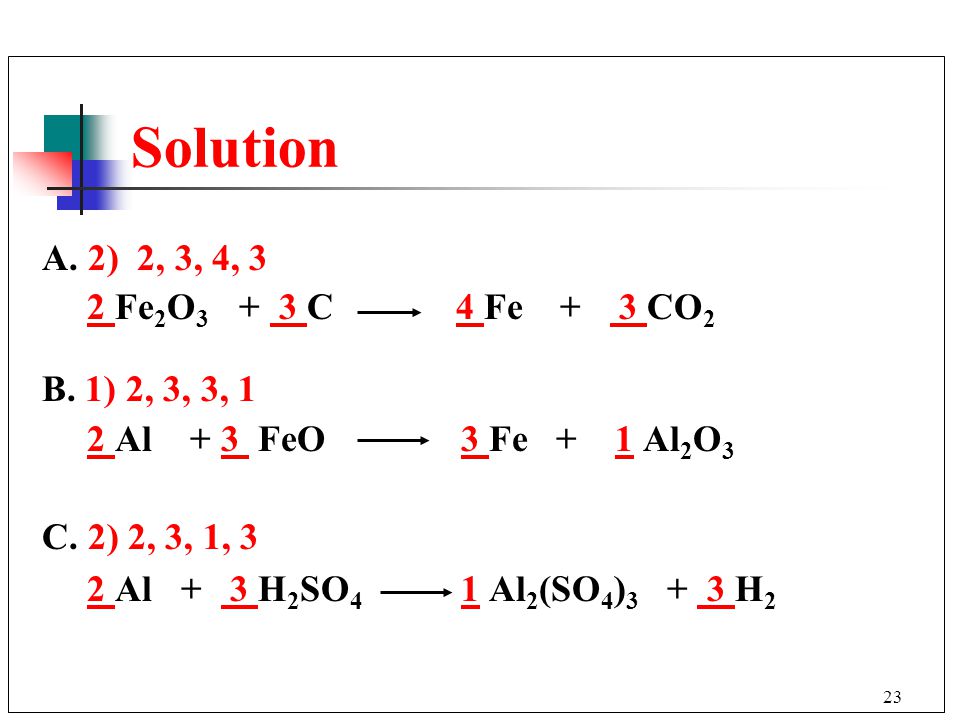
balance the chemical equation :-1. feO3+CO → Fe + CO22. NH3+O2 → NO+H2O3. Fe + H2O → Fe3O4+H24. - Brainly.in

PDF) Heat transfer and chemical reaction models for reduction of iron oxide pellets in countercurrent moving bed reactors

Microstructure and catalytic properties of Fe3O4/BN, Fe3O4(Pt)/BN, and FePt/BN heterogeneous nanomaterials in CO2 hydrogenation reaction: Experimental and theoretical insights - ScienceDirect

PDF) TG/DTA study on the carbon monoxide and graphite thermal reduction of a high-grade iron nickel oxide residue with the presence of siliceous gangue


.jpg)