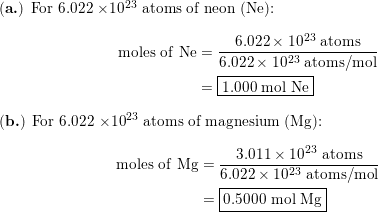Which of the following contains the same number of atoms as 4.032g of hydrogen atoms? A. 1 mole of H2 - Brainly.com
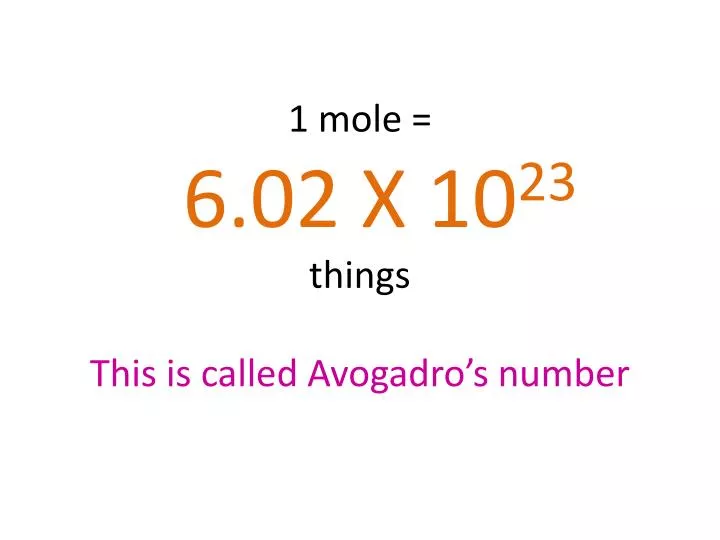
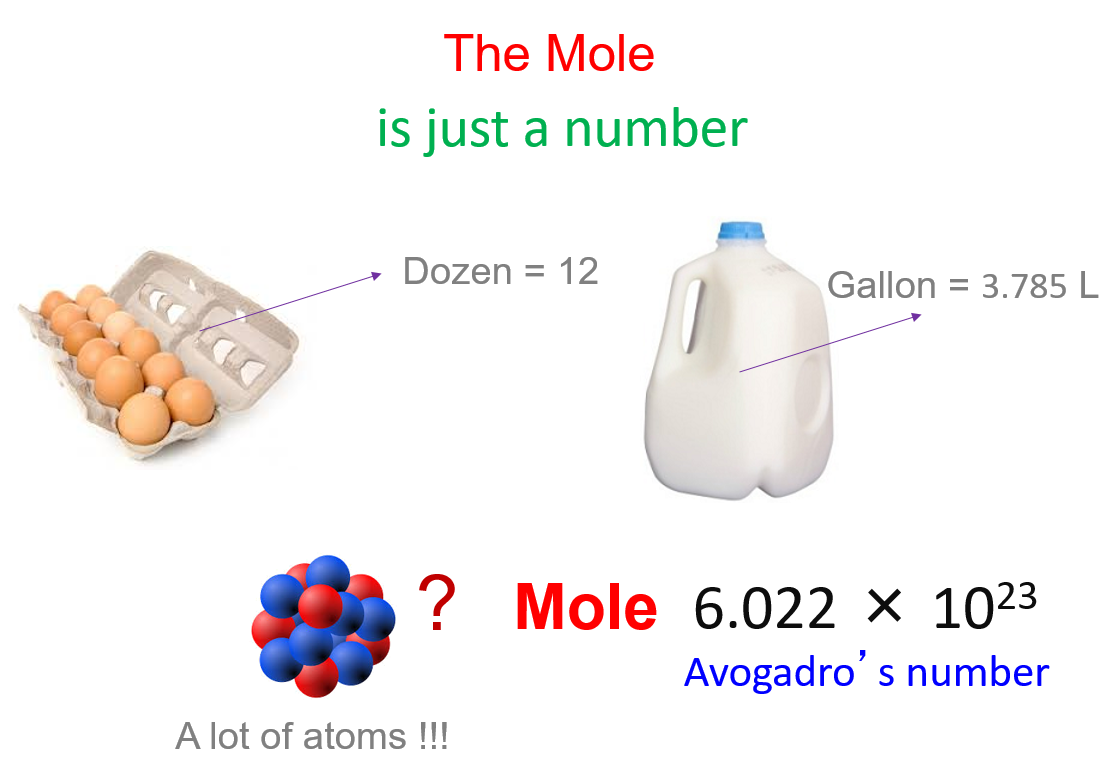
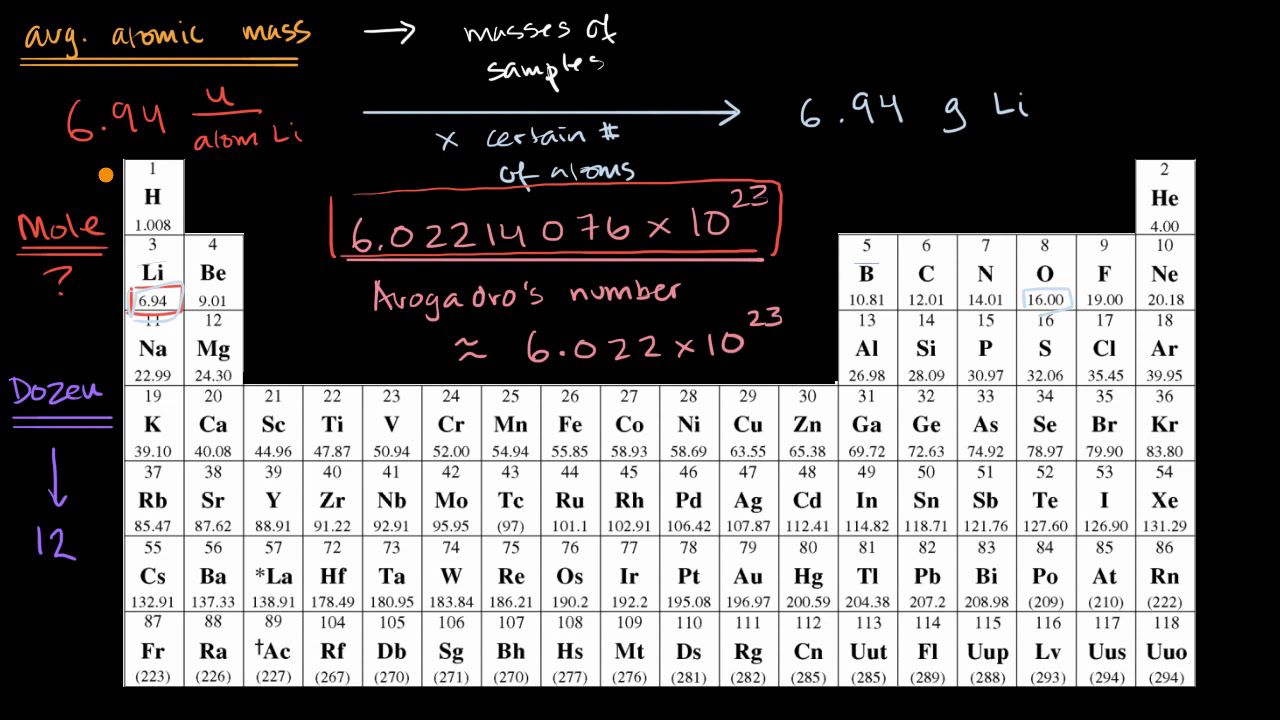
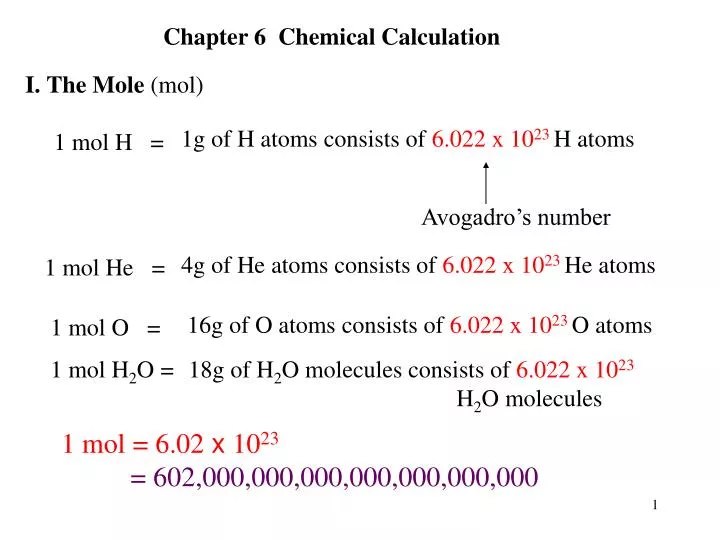
PPT - 1 mole = 6.02 X 10 23 things This is called Avogadro's number PowerPoint Presentation - ID:4272623

Question Video: Determining the Number of Oxygen Atoms Present in a Given Number of Moles of Aluminum Nitrate | Nagwa

1 mole = 6 022 x 10^23 If there is 1 mole of H2 we have multiply the Avogadro no - Science - Atoms and Molecules - 15776529 | Meritnation.com

12 g C - 12 contains 6.022 × 10^23 atoms of carbon.(a) 6.022 × 10^23 is known as .............(b) Calculate the number of carbon atoms present in 48 g C - 12.(c)